How To Find Internal Energy
The internal energy of steam(wet steam) is illustrated by the image below.
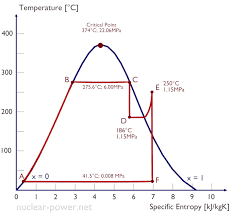
To compute for internal free energy of steam, v essential parameters are needed and these parameters are Enthalpy of Wet Steam (h), Specific Volume of Gas (5g), Pressure (P), Dryness Fraction (x) and J.
The formula for calculating the internal energy of steam(wet steam):
U = h –Pxvg /J
Where:
U = Internal Energy of Steam(wet Steam)
h = Enthalpy of Wet Steam
vg = Specific Book of Gas
x = Dryness Fraction
P = Pressure
Given an example;
Find the internal free energy of steam (wet steam) when the enthalpy of moisture steam is 16, the specific volume of gas is 13, the dryness fraction is x and the pressure is 6.
This implies that;
h = Enthalpy of Moisture Steam = 16
vg = Specific Volume of Gas = 13
x = Dryness Fraction = 10
P = Pressure = half-dozen
U = h –Pxvthousand /J
U = sixteen –ten x vi ten 13 /one
U = xvi –780 /1
U = 16 – 780
U = -764
Therefore, theinternal energy of steam (moisture steam)is-764 J.
Computing the Enthalpy of Wet Steam when the Internal Energy of Steam, the Specific Volume of Gas, the Dryness Fraction and the Force per unit area is Given.
h = U + Pxv1000 / J
Where:
h = Enthalpy of Moisture Steam
U = Internal Energy of Steam(moisture Steam)
fiveg = Specific Volume of Gas
10 = Dryness Fraction
P = Pressure
Allow'south solve an example;
Detect the enthalpy of wet steam when the internal energy of steam is 14, the specific book of gas is 2, the dryness fraction is 6 and the pressure is 3.
This implies that;
U = Internal Energy of Steam(moisture Steam) = xiv
fivegrand = Specific Volume of Gas = 2
ten = Dryness Fraction = 6
P = Pressure = 3
h = U + Pxvk / J
h = 14 + iii(vi)(two) / one
h = fourteen + 36 / 1
h = 14 + 36
h = 50
Therefore, the enthalpy of wet steam isfifty.
Calculating the Specific Volume of Gas when the Internal Energy of Steam, the Enthalpy of Moisture Steam, the Dryness Fraction and the Pressure is Given.
fiveyard = h – U / Px x J
Where:
fiveg = Specific Volume of Gas
U = Internal Free energy of Steam(moisture Steam)
h = Enthalpy of Wet Steam
x = Dryness Fraction
P = Force per unit area
Allow's solve an example;
Find the specific volume of gas when the internal energy of steam is 9, the enthalpy of moisture steam is 18, the dryness fraction is 7 and the pressure is ii.
This implies that;
U = Internal Energy of Steam(wet Steam) = ix
h = Enthalpy of Wet Steam = 18
x = Dryness Fraction = 7
P = Pressure = 2
vg = h – U / Px x J
fivethousand = 18 – 9 / vii(ii) x ane
vm = nine / fourteen ten 1
fivek = 0.64 x i
fiveg = 0.64
Therefore, thespecific book of gasis0.64.
Computing the Dryness Fraction when the Internal Energy of Steam, the Enthalpy of Wet Steam, the Specific Volume of Gas and the Pressure level is Given.
ten = h – U / Pvthousand x J
Where:
x = Dryness Fraction
U = Internal Free energy of Steam(wet Steam)
h = Enthalpy of Wet Steam
vone thousand = Specific Volume of Gas
P = Force per unit area
Permit's solve an case;
Find the dryness fraction when the internal energy of steam is half dozen, the enthalpy of wet steam is 24, the specific volume of gas is four and the pressure is two.
This implies that;
U = Internal Energy of Steam(moisture Steam) = 6
h = Enthalpy of Wet Steam = 24
5thou = Specific Volume of Gas = 4
P = Pressure = ii
10 = h – U / Pvg x J
ten = 24 – 6 / ii(iv) x i
x = 18 / 8 x 1
x = ii.25 10 1
x = 2.25
Therefore, thedryness fractionis2.25.
Calculating the Pressure level when the Internal Energy of Steam, the Enthalpy of Moisture Steam, the Specific Volume of Gas and the Dryness Fraction is Given.
P = h – U / xvg x J
Where:
P = Force per unit area
U = Internal Free energy of Steam(moisture Steam)
h = Enthalpy of Wet Steam
vone thousand = Specific Volume of Gas
x = Dryness Fraction
Let'due south solve an example;
Discover the pressure when the internal energy of steam is x, the enthalpy of moisture steam is 32, the specific volume of gas is 5 and the dryness fraction is 6.
This implies that;
U = Internal Free energy of Steam(wet Steam) = 10
h = Enthalpy of Wet Steam = 32
fiveg = Specific Volume of Gas = 5
x = Dryness Fraction = 6
P = h – U / xvone thousand x J
P = 32 – 10 / 6(five) x 1
P = 22 / 30 ten i
P = 0.73 x 1
P = 0.73
Therefore, thepressure levelis0.73.
Nickzom Estimator –The Calculator Encyclopedia is capable of calculating the internal energy of steam (wet steam).
At present, Click onChemic Reaction Thermodynamics netherMaterials and Metallurgical
Now, Click onEnthalpy netherChemical Reaction Thermodynamics
At present, Click on Internal Energy of Steam (wet steam) underEnthalpy
The screenshot below displays the folio or activity to enter your values, to get the answer for the internal energy of steam according to the respective parameter which is the Enthalpy of Wet Steam (h), Specific Volume of Gas (vg), Pressure (P), Dryness Fraction (x) and J.
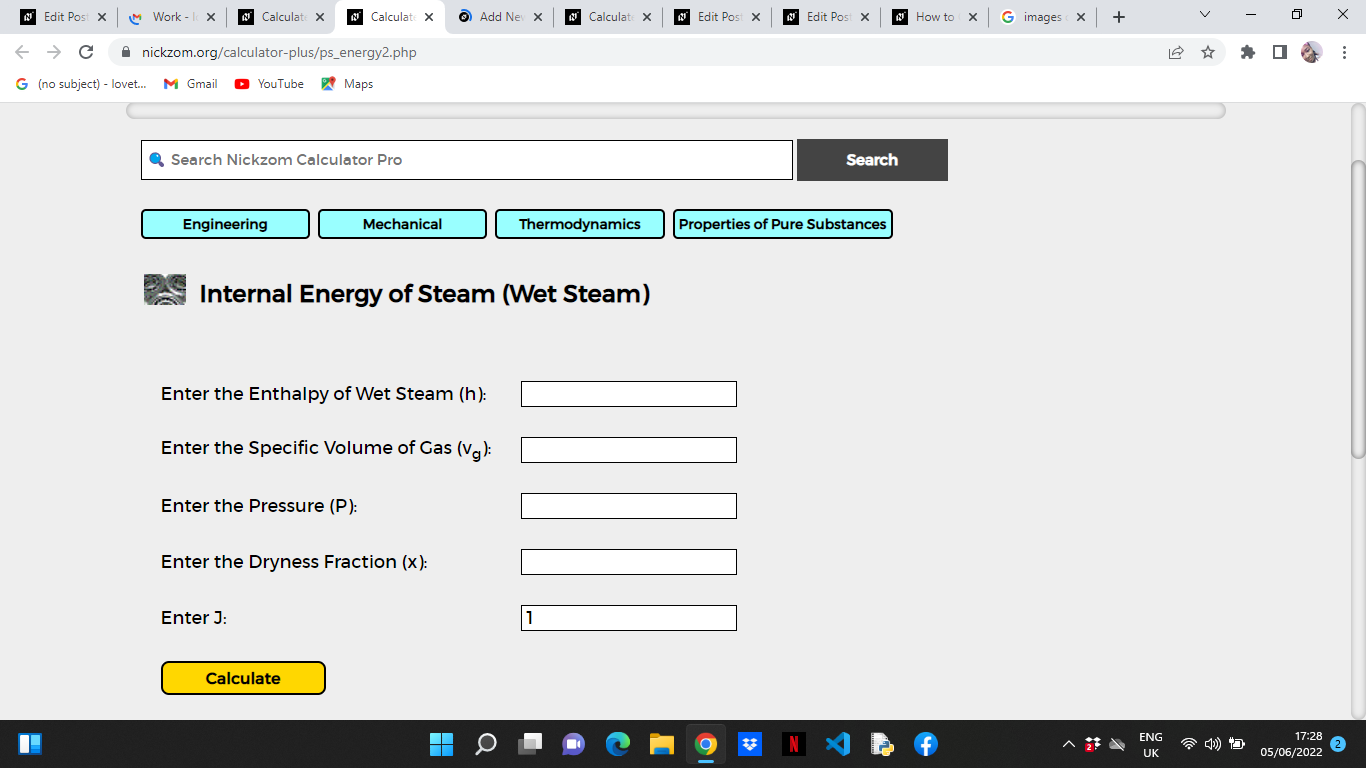
Now, enter the values appropriately and accordingly for the parameters as required by the Enthalpy of Wet Steam (h) is sixteen, Specific Book of Gas (5g) is 13, Pressure (P) is x, Dryness Fraction (10) is 6 and J is 1.
Finally, Click on Summate
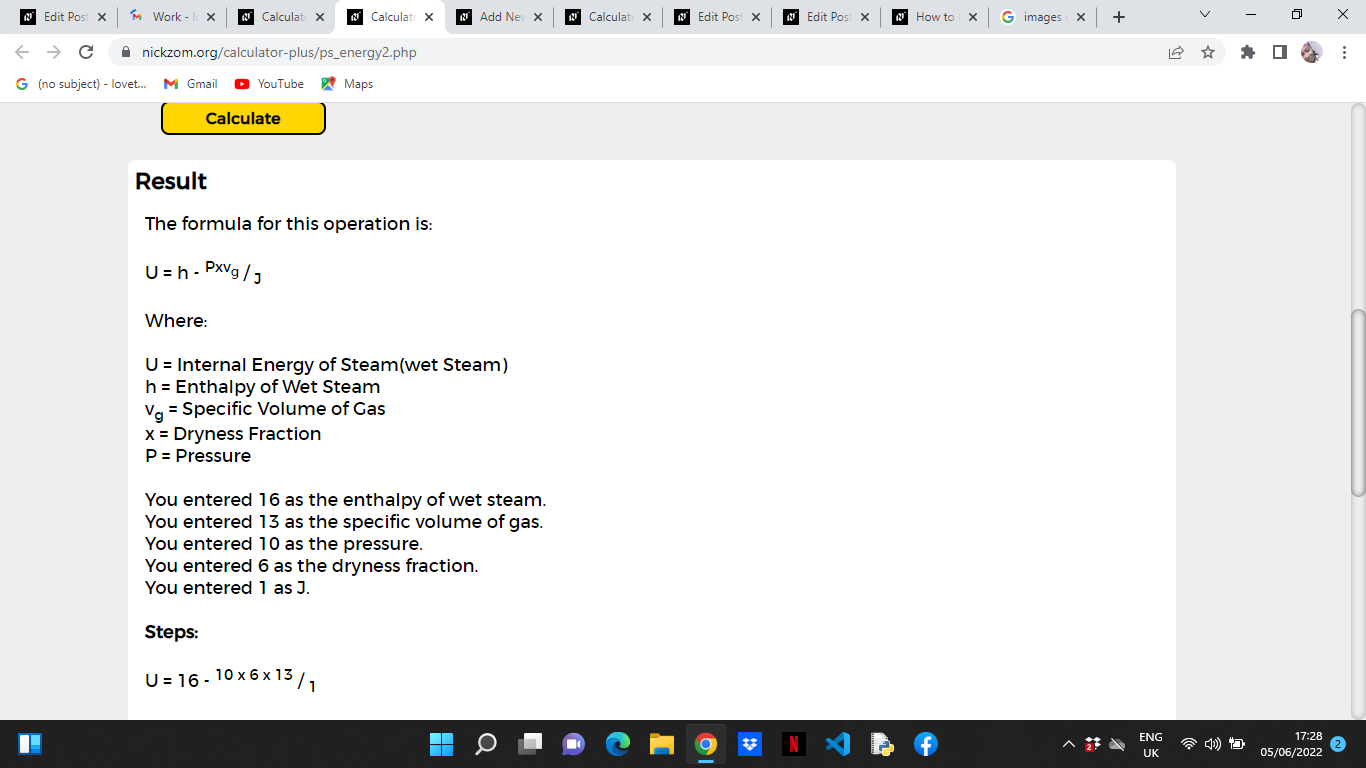
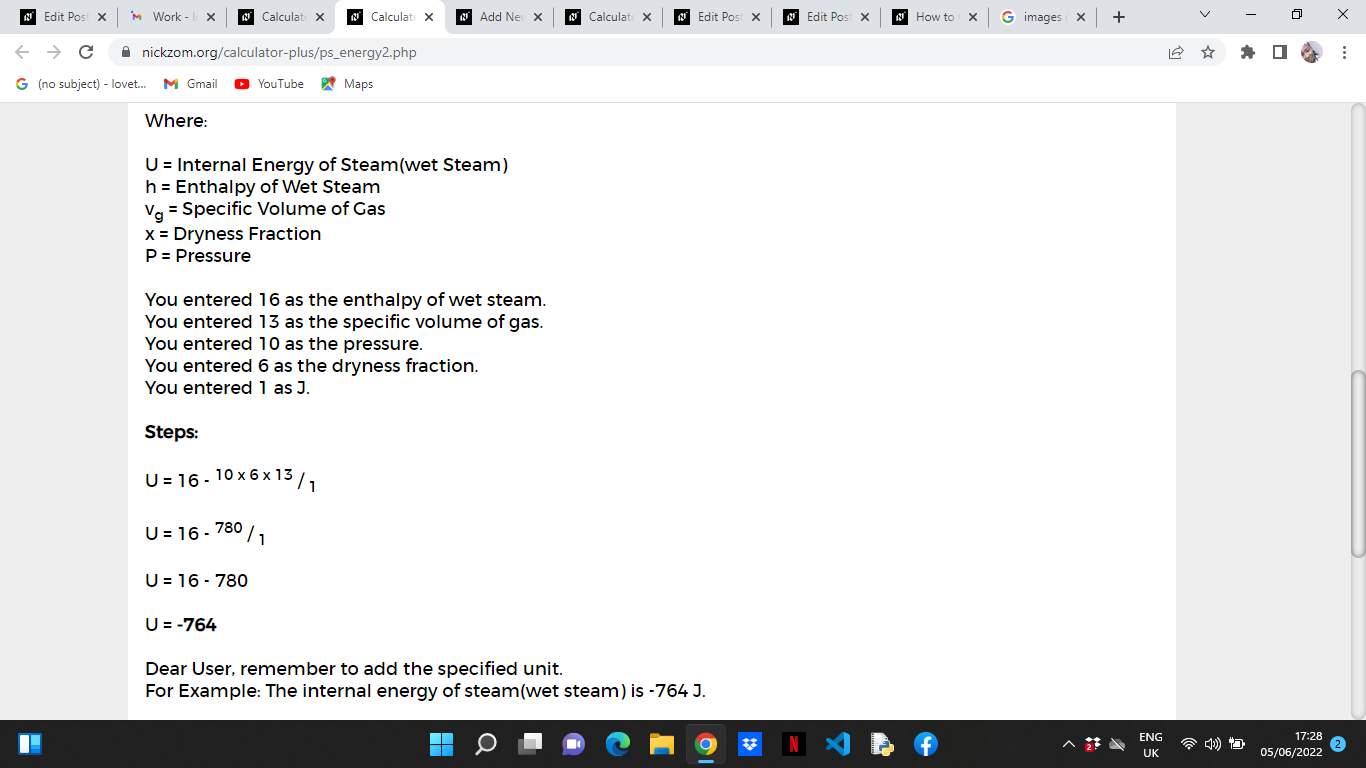
As you can see from the screenshot above,Nickzom Calculator– The Calculator Encyclopedia solves for the internal energy of steam and presents the formula, workings and steps too.
How To Find Internal Energy,
Source: https://www.nickzom.org/blog/2022/06/06/how-to-calculate-and-solve-for-internal-energy-of-steam-wet-steam-enthalpy/
Posted by: ablerithey.blogspot.com


0 Response to "How To Find Internal Energy"
Post a Comment